
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
Bis(thiomethyl)- and bis(thiohexyl)-tetrathiafulvalene-bromo-benzothiadiazoles, containing electron donor tetrathiafulvalene (TTF) and electron acceptor benzothiadiazole (BTD) units, have been prepared by Stille coupling reactions between the TTF-SnMe3 precursors and BTD-Br2. In another series of experiments, TTF-acetylene-BTD compounds have been synthesized by Sonogashira coupling between either TTF-acetylenes and BTD-Br2 in low yields, or TTF-iodine and BTD-acetylene in moderate yields. In the compound TTF-C≡C-BTD the TTF and BTD units are coplanar in the solid state, as shown by the single crystal X-ray structure, and there is segregation in the packing between the donor and acceptor units. All the derivatives have good electron donor properties, as determined by cyclic voltammetry measurements, and they can also be reversibly reduced thanks to the presence of the BTD moiety. UV-visible spectroscopy and photophysical investigations show the presence of an intramolecular charge transfer (ICT) band and an emission band originating from the charge transfer. Both the absorption and the emission are modulated by the substitution scheme and the insertion of the acetylenic bridge. | ||||||||
 |
|
|||||||
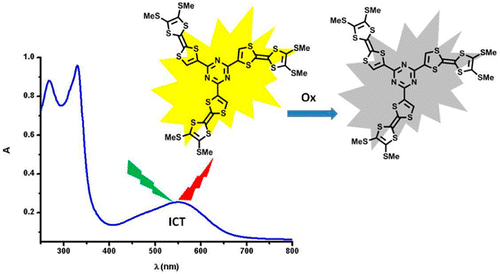
Palladium-catalyzed cross-coupling reactions between chlorinated 1,3,5-triazines (TZ) and tetrathiafulvalene (TTF) trimethyltin derivatives afford mono- and C3 symmetric tris(TTF)-triazines as donor–acceptor compounds in which the intramolecular charge transfer (ICT) is modulated by the substitution scheme on TTF and TZ and by chemical or electrochemical oxidation. The TTF-TZ-Cl2 and (SMe)2TTF-TZ-Cl2 derivatives show fully planar structures in the solid state as a consequence of the conjugation between the two units. Electrochemical and photophysical investigations, supported by theoretical calculations, clearly demonstrate that the lowest excited state can be ascribed to the intramolecular charge transfer (ICT) π(TTF)→π*(TZ) transition. The tris(TTF) compound [(SMe)2TTF]3-TZ shows fluorescence when excited in the ICT band, and the emission is quenched upon oxidation. The radical cations TTF+• are easily observed in all of the cases through chemical and electrochemical oxidation by steady-state absorption experiments. In the case of [(SMe)2TTF]3-TZ, a low energy band at 5000 cm–1, corresponding to a coupling between TTF+• and TTF units, is observed. A crystalline radical cation salt with the TTF-TZ-Cl2 donor and PF6– anion, prepared by electrocrystallization, is described. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017
